
Chiral molecules can take two configurations, called enantiomers, whose mirror images of each other are not overlapping. If their physicochemical properties are identical, the two enantiomers of a molecule, for example a drug, can have very different physiological effects or even antagonists. Being able to detect them individually, separate them or determine their proportions in a solution is therefore essential. Researchers from ISM (CNRS / University of Bordeaux / Bordeaux INP) and CRPP (CNRS / University of Bordeaux), with their Italian partners, propose an extremely simple and novel approach, based on miniaturized swimmers, which is published in the journal Nature Chemistry.
Hands, shoes, snail shells, certain plants … are chiral objects. A molecule is chiral if it has two forms, said to be enantiomeric, whose images of each other in a mirror cannot be superimposed. Almost all the physico-chemical properties of two enantiomers are identical (mass, melting point, solubility…). On the other hand, many physiological functions (perception of odors, metabolism, etc.) are based on the specific recognition of only one of the two enantiomers. The pharmaceutical properties of two enantiomers can therefore be very different, one being beneficial, the other ineffective or even toxic. If, as early as 1848, Pasteur succeeded in distinguishing the two enantiomers of a chemical compound by hand sorting the crystals of each enantiomer, significant efforts continue to be made today by scientists to develop methods which make it possible to detect and / or sorting enantiomers selectively.
Several approaches are currently used, in particular by optical, chromatographic or electrochemical means, which require more or less complex equipment. Researchers from the Institute of Molecular Sciences of Bordeaux (CNRS / University of Bordeaux / Bordeaux INP) and the Paul Pascal Research Center (CNRS / University of Bordeaux) have just proposed, with their Italian colleagues, a new approach which allows a very simple analysis of a solution containing chiral molecules using miniaturized swimmers. These objects, made from a conductive polymer, are modified on one side by a deposition of enzymes and on the other end by chiral oligomers. The driving force behind propelling these swimmers to the air / water interface comes from the enzymatic conversion of oxygen. The trajectory, and in particular the direction of rotation of these swimmers, depends on the chirality of the molecules present in solution which interact with the chiral oligomers grafted on the swimmer. One enantiomer will induce a gyratory movement in a clockwise direction, while the other causes a rotation in the opposite direction. The analysis of the trajectory even allows us to go back to the relative concentration of each enantiomer. These results, obtained within the framework of the ERC Advanced ELECTRA project, pave the way for a whole new family of analytical methods to determine the chiral character of a solution in an extremely simple and rapid manner, unlike the approaches currently used.
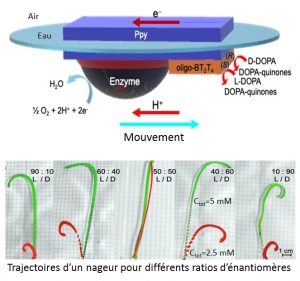
© Alexander Kuhn
Direct dynamic readout of molecular chirality with autonomous enzyme driven swimmers
Serena Arnaboldi, Gerardo Salinas, Aleksandar Karajić, Patrick Garrigue, Tiziana Benincori, Giorgia Bonetti, Roberto Cirilli, Sabrina Bichon, Sébastien Gounel, Nicolas Mano, Alexander Kuhn, Nature Chemistry 14 octobre 2021.
https://doi.org/10.1038/s41557-021-00798-9
Researcher Contact
Alexander Kuhn (Laboratory ISM)
