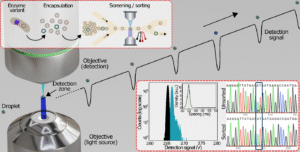
Absorption-based high-throughput microfluidic screening using ultrafine aqueous droplets is at the forefront of enzyme engineering. Yet at the micron scale, light absorption is weak, and scattering from the droplet surface reduces the detection sensitivity. As a result, measurements often require larger droplets or longer acquisition times, limiting throughput to below 1 kHz. A team of researchers from the CRPP (Centre de Recherche Paul Pascal, CNRS/Université de Bordeaux) addressed these challenges and demonstrated a way to push past the limits of existing absorbance-based high-throughput droplet screening systems, by combining a confocal detection scheme with a microfluidic high-precision droplet control. Published in Advanced Science, their findings contribute to advances in microfluidic-directed evolution and other ultrahigh-throughput biochemical applications.
Enzymes are essential catalysts in biological and industrial processes, but their natural performance which is shaped by billions of years of evolution rarely matches specific laboratory or industrial needs. Directed Evolution addresses this gap by recreating evolution on a lab scale, iteratively mutating, and selecting enzymes to meet user-defined requirements. The key challenge identifying target variants from millions of sequences. Conventional screening methods based on color or fluorescence changes in agar or microtiter plates are too slow for such large libraries. The use of microfluidics has been demonstrated to accelerate the process by confining reactions inside picoliter-sized water-in-oil droplets, thus enabling screening at a rate substantially faster than conventional strategies. While most droplet-based studies to date have relied on fluorescence readouts, it is important to note that many significant enzymes lack suitable fluorescent assays. Absorbance-based detection has been shown to be compatible with a wide range of chromogenic assays, thus offering a valuable alternative. Nevertheless, this method is constrained by the presence of scattering artifacts and a comparatively low throughput.
The CRPP team addressed these limitations by developing a confocal Absorbance-Activated Droplet Sorting (cAADS) system. Their platform enables sensitive absorbance measurements at ultrahigh throughput (5.4 kHz) from droplets as small as 10 pL, which is 3.6 times faster than the most advanced absorbance-based screening. Using 50 pL droplets, they demonstrated dielectrophoretic sorting at frequencies up to 2.6 kHz, reaching performance comparable to gold-standard fluorescence-based systems. The team validated their approach by enriching active variants of Bilirubin Oxidase (BOD) with 99% sorting efficiency and by screening glucose oxidase (GOx) to demonstrate the versatility of the system. Together, these results establish cAADS as a powerful new tool for absorbance-based microfluidic screening, with broad potential in enzyme engineering and beyond.
Reference
Abdi Mirgissa Kaba, Sébastien Gounel, Thomas Beneyton, Lionel Buisson, Jean-Christophe Baret, and Nicolas Mano.
Confocal Absorbance-Activated Droplet Sorting (cAADS) for Enzyme Engineering Adv. Sci. 2025 https://doi.org/10.1002/advs.202505324
Contact
Nicolas Mano, Chercheur au Centre de recherche Paul Pascal (CNRS/Université de Bordeaux)
Jean-Christophe Baret, Enseignant-chercheur au Centre de recherche Paul Pascal (CNRS/Université de Bordeaux)
jean-christophe.baret@u-bordeaux.fr
